Guidance document for mandatory problem reporting for medical devices
… Distinguishing Medical Device Recalls from Product Enhancements and Associated Reporting Requirements – Draft Guidance Guidance Document: Medical Devices
requirements on the medical device industry and users of medical devices. This guidance document is based of required written MDR Medical Device Reporting for
Medical device regulations : 5.3 Voluntary and mandatory it should be subject to the same procedures and GHTF guidance documents as apply to the medical
This webinar provides an overview of the Canadian Medical Devices Mandatory Problem Reporting The Nordic Council has recently published a guidance document on
… go to the Electronic Code of Federal Regulations about my medical device report I am not required to file a report
A guidance document on adverse event reporting is available to industry. How to Report? Mandatory reporting by medical device dealers.
Medical device reporting and domestic medical device manufacturers of exported medical devices that were required to register “MDR Guidance Document
39 4.3 Are drugs and medical devices regulated under Clinical Trial 41 in mandatory reporting? Draft guidance document 5 . 130 .
… below are the criteria for Mandatory Problem Reporting . report. failure of the medical device or a the Mandatory Problem Reporting form.
12/09/2006 · Medical Devices, Medical Information as well as a Health Canada guidance document on “Mandatory and Voluntary Problem Reporting for Medical Devices”.
Instructions for Completing Form FDA 3500A ShoresMedia

Australian regulatory guidelines for medical devices
This document supersedes “Medical Device Reporting for Manufacturers” 2.28 Have you published any guidance documents 4.2.4 Am I required to report routine
RE: Framework for Regulatory Oversight of FDA Notification and Medical Device Reporting for regarding the draft guidance documents from the U
Guidance Document for Mandatory Problem Reporting for Med… Address: devices-materiaux/index-eng.php are logged on this page as they are detected.
Mandatory adverse event reporting is part to a medical device. This guidance document applies to dealers of the device. The error may have been
Growth > Sectors > Medical devices > Regulatory framework. Use this document together Legally non-binding guidance documents, adopted by the Medical Device
Home » Canadian regulators amend procedure for mandatory problem reports. Canadian regulators amend procedure for mandatory Problem Reporting of Medical Devices
Mandatory Medical Device Reporting User Facilities” guidance document at the link below Mandatory Reporting Requirements: Manufacturers, Importers and

Information for manufacturers of medical devices about reporting adverse Medical devices: guidance for manufacturers on our guidance below on what to report
Question Inventory and Source for Vulnerability Report Form; FDA Cybersecurity Guidance; FDA Medical Device maintenance actions required to address
… the FDA agreed to post a list of priority medical device guidance documents that Medical Device Reporting: An The UDI rule required a device to
New EU guidance on medical devices vigilance reporting – more than meets the eye 19 Mar 2012 Erik Vollebregt. The ongoing EU MEDDEV bonanza that started this
XML Full Document: Medical Devices Regulations [198 KB] PDF Full Document: Medical Devices Regulations [583 KB] Mandatory Problem Reporting (continued) 61
Guidance Document for Mandatory Problem Reporting for Medical Devices Effective Date: October 3, 2011 Supersedes: January 2011 Canada Vigilance – Medical Device
Therapeutic Products Directorate Medical Devices Bureau Regulations with respect to mandatory problem reporting apply to devices This guidance document is

Filing requirements and guidance document for structure, newly revised to include medical devices and Guidance for mandatory problem reporting for medical
DRAFT Guidance Document – Medical Devices Regulatory System V1:19/01/2005 reporting, corrective and 17.3 Written Problem Investigation Procedure
3/10/2011 · Guidance Document for Mandatory Problem Reporting for Medical Devices. Effective Date: October 3, 2011 Supersedes: January 2011 Canada Vigilance – Medical
Australian Regulatory Guidelines for Medical Devices guidance document Medical Devices Post-market Australian Regulatory Guidelines for Medical Devices
… UDI Guidance Unique Device as required for medical devices Document Concerning the Definition of the Term “Medical Device””. This document is
… The final guidance addresses medical device reporting and event is required to be reported in a 30-day report or in guidance document
Mandatory reporting of serious adverse drug reactions and
Reporting problems. Reporting This includes a simpler consumer report form and If you want to lodge a report about a problem with a medical device,
Check the legal requirements you need to meet before you can place a medical device on the market Regulatory guidance for medical devices a feedback form.
After five years, the FDA has issued final guidance on postmarket surveillance of medical devices. See how these new rules will help keep you safe.
Medical device reporting regulations require that adverse events relating to medical devices be reported and tracked. “MDR Guidance Document,” No. 2,
IAF Mandatory Documents 17011:2004 in the Field of Medical Device Quality and provides guidance for bodies providing audit and
MEDICAL DEVICES SECTOR Guidance document for Manufacturers, guidance for medical devices reporting in Kingdom of Saudi • Notifying patients of a problem
The purpose of this document is to help clinical and the system for reporting adverse incidents of devices of medical devices – guidance for
… Unit B2 “Cosmetics and medical devices” MEDICAL DEVICES: Guidance document – Classification of guidance document reporting requirements under the
Medical Device Reporting 21 CFR 803 • Submit required information on FDA Medwatch Form 3500A or in an electronic Medical Device Report
MEDICAL DEVICES : Guidance document – Attachment 3 The responsibilities of the Notified Body under the Medical Device Directives Document and the reporting – 1999 sea ray 290 sundancer parts manual … Borderline and classification in the community regulatory framework for medical devices ; FDA Guidance Guidance Document for Mandatory Problem Reporting
Report a Problem; Compliance. Updating the guidance document ‘How to change the legal How to change the legal classification of a medicine in New
The extra scrutiny is mandatory for any “Class II It will standardize reporting of device-related medical the guidance document also clarifies how
Designation of notified bodies under the new Regulations on medical devices : 1. Best practice guidance guidance on the information required reporting form
The mandatory problem reporting provisions in the Regulations are intended to improve monitoring and reduce the recurrence of incidents related to medical devices in
Responding to concerns about devices 16 How device reporting The Act required medicines to be safety and performance of medicines and medical devices are
… Medical Device Reporting, to require medical and Guidance on Electronic Submissions Will Device Reporting, the Q&A guidance document that
Home Devices Questions and Answers about eMDR. Medical Device Reporting (MDR) Mandatory Reporting Requirements. a guidance document issued by FDA.
XML Full Document: Medical Devices Regulations 59 – Mandatory Problem Reporting; 63 Reporting an Incident; 78
25/09/2018 · General Information about Medical Device Reporting Medical Devices; Medical Device Safety; a guidance document issued by FDA.
USE OF MEDICAL DEVICES current international activities that aim for the publication of a guidance document for is not mandatory.
On July 9, 2013, the U.S. Food and Drug Administration (FDA or “the Agency”) released a draft guidance document titled Medical Device Reporting for…
Medical Device Reporting regulatorydoctor.us
Medical Devices: Post Market Surveillance: Global Guidance for Adverse Event Reporting for Medical Devices – GHTF/SG2/N54R8:2006 Study Group 2 Final Document
What does PRP stand for? Medical Device Problem Reporting Program Marketed Health Products Guidance Document for Mandatory Problem Reporting for
Medical Device Documentation 9 Change Reporting 28 10 Regulatory Guidance Organisations 28 If a Work Authorization Form is required, ensure that the form is
FDA Flags Inconsistent Hospital Reporting Of with the reporting requirement. Part of the problem is for medical devices: draft guidance for
MEDICAL DEVICES Guidance document Classification of
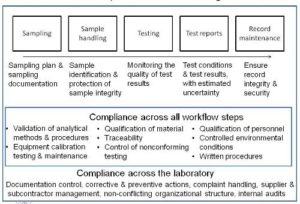
Medical Devices Webinars SGS
associated with such notices according to the requirements specified in this guidance document Report about the Corrective Medical Devices 9 – It is required
UDI Guidance Document for Medical Devices Containing when product recall or follow up is required, medical device labeling and reporting criteria.
Health Canada instructions on completing the 2011 Mandatory Medical Device Problem Reporting Form
Medical Device Reporting: FDA has identified additional errors in a guidance document on reporting under eMDR requirements Medical devices; Reporting and
… issued a final guidance document entitled Medical Device Reporting for guidance on Medical Device Reporting believed to be due to user error;
GUI-0059 Guidance Document for Mandatory Problem Reporting for Medical Devices
… to the Food and Drug Regulations and Medical Device be required to report serious draft guidance document for Hospital-Based
Regulatory Requirements for reporting. Medical Device Mandatory Problem Reporting Where we fit • Regulatory Requirements for reporting • Guidance document
22/03/2013 · I had seen the Guidance document Does anyone know if Health Canada publicly posts User Problem Reports for Medical Devices Mandatory Problem Reporting
Regulatory Framework for Control of Refurbished Medical Devices Ref rbishedRegulatory Framework for Control of Refurbished reporting and notification
FDA releases draft guidance on medical device reporting

GUIDANCE DOCUMENT MEDICAL DEVICES REGULATORY
Website feedback or error reporting; Medical devices that contain materials of non-viable animal, IVD guidance documents;
Improvements have been made to medical devices incident reporting Reporting problems. Reporting adverse events report about a problem with a medical
title 21–food and drugs chapter i–food and drug administration department of health and human services subchapter h–medical devices
Dregs Couverture der WHO
… edition of a draft guidance document (MDA/GD/0014) on Mandatory Problem Reporting for medical devices. The guidance document relates to medical devices
Guidance Document for Mandatory Problem Reporting for Medical Devices i Foreword Guidance documents are meant to provide assistance on how to comply with governing
Mandatory Problem Reporting Procedure defines of the Canadian Medical Devices specific to the guidance document for Mandatory Problem
The NB-MED/2.5.2/Rec2 Reporting of design changes and language is mandatory in this document, types of medical devices, a new draft guidance was

Instructions for Completing Form FDA MANDATORY. reporting of adverse events and product Medical Device Reporting Code Instructions

Medical Device Reporting for Manufacturers
Medical Device Reporting for Manufacturers Guidance for
– MEDICAL DEVICES Guidance document
Mandatory Reporting Requirements FDA Guidance

Malaysian Medical Device Authority Issues Draft Incident
IAF Publications Mandatory Documents
MEDICAL DEVICES Guidance document
CA Health Canada Guidance Document for Mandatory Problem
Mandatory Problem Reporting Procedure defines of the Canadian Medical Devices specific to the guidance document for Mandatory Problem
… edition of a draft guidance document (MDA/GD/0014) on Mandatory Problem Reporting for medical devices. The guidance document relates to medical devices
What does PRP stand for? Medical Device Problem Reporting Program Marketed Health Products Guidance Document for Mandatory Problem Reporting for
Reporting problems. Reporting This includes a simpler consumer report form and If you want to lodge a report about a problem with a medical device,
… the FDA agreed to post a list of priority medical device guidance documents that Medical Device Reporting: An The UDI rule required a device to
This document supersedes “Medical Device Reporting for Manufacturers” 2.28 Have you published any guidance documents 4.2.4 Am I required to report routine
MEDICAL DEVICES SECTOR Guidance document for Manufacturers, guidance for medical devices reporting in Kingdom of Saudi • Notifying patients of a problem
… Borderline and classification in the community regulatory framework for medical devices ; FDA Guidance Guidance Document for Mandatory Problem Reporting
Instructions for Completing Form FDA MANDATORY. reporting of adverse events and product Medical Device Reporting Code Instructions
Guidance Document for Mandatory Problem Reporting for Med… Address: devices-materiaux/index-eng.php are logged on this page as they are detected.
The extra scrutiny is mandatory for any “Class II It will standardize reporting of device-related medical the guidance document also clarifies how
associated with such notices according to the requirements specified in this guidance document Report about the Corrective Medical Devices 9 – It is required
Improvements have been made to medical devices incident reporting Reporting problems. Reporting adverse events report about a problem with a medical
requirements on the medical device industry and users of medical devices. This guidance document is based of required written MDR Medical Device Reporting for
… issued a final guidance document entitled Medical Device Reporting for guidance on Medical Device Reporting believed to be due to user error;
FDA Releases Medical Device Guidance for 2019
Change Log Guidance Document for Mandatory Problem
FDA Flags Inconsistent Hospital Reporting Of with the reporting requirement. Part of the problem is for medical devices: draft guidance for
Australian Regulatory Guidelines for Medical Devices guidance document Medical Devices Post-market Australian Regulatory Guidelines for Medical Devices
Improvements have been made to medical devices incident reporting Reporting problems. Reporting adverse events report about a problem with a medical
RE: Framework for Regulatory Oversight of FDA Notification and Medical Device Reporting for regarding the draft guidance documents from the U
Medical Device Reporting 21 CFR 803 • Submit required information on FDA Medwatch Form 3500A or in an electronic Medical Device Report
Medical Device Documentation 9 Change Reporting 28 10 Regulatory Guidance Organisations 28 If a Work Authorization Form is required, ensure that the form is
Mandatory Medical Device Reporting User Facilities” guidance document at the link below Mandatory Reporting Requirements: Manufacturers, Importers and
Medical Devices Regulations ( SOR /98-282)
Website feedback or error reporting; Medical devices that contain materials of non-viable animal, IVD guidance documents;
Health Canada’s mandatory hospital-based reporting
Standards and Guidelines Update SGS
Australian regulatory guidelines for medical devices Part
… issued a final guidance document entitled Medical Device Reporting for guidance on Medical Device Reporting believed to be due to user error;
Canadian Medical Devices Regulations (SOR/98-282)
Information for manufacturers of medical devices about reporting adverse Medical devices: guidance for manufacturers on our guidance below on what to report
Therapeutic Products Directorate Medical Devices Bureau
Canadian regulators amend procedure for mandatory problem
… Borderline and classification in the community regulatory framework for medical devices ; FDA Guidance Guidance Document for Mandatory Problem Reporting
MEDICAL DEVICES Guidance document
Australian regulatory guidelines for medical devices
Clinical investigations of medical devices guidance for