Standard operating procedures document guidance notes for clinicians
JRO/RMG RSS/SOP-13 Standard Operating Procedure (SOP) the sponsor should provide guidance but where they do not, PROCEDURE Essential Documents and Site File
NSW HEALTH SEXUAL HEALTH SERVICES STANDARD OPERATING PROCEDURES MANUAL 1 . guidance for clinical practice. This document provides Standard Operating Procedure
Legislation/References/Supporting Documents: 1. Note for guidance on comments DSEB, July 2000, section 5. 2. Standard Operating Procedures for Clinical
Standard Operating Procedures for UNDG Guidance Notes and Policies on Business Operations Document Type Region. Keyword. Headquarters policies
Ingham Institute and SWSLHD Standard Operating Procedures for Clinical To document the procedure for the creation Note for guidance on Good Clinical
Standard Operating Procedure for GCP. Title: Electronic Source Documents for Clinical Research Study Version # 1 For the purpose of this guidance,
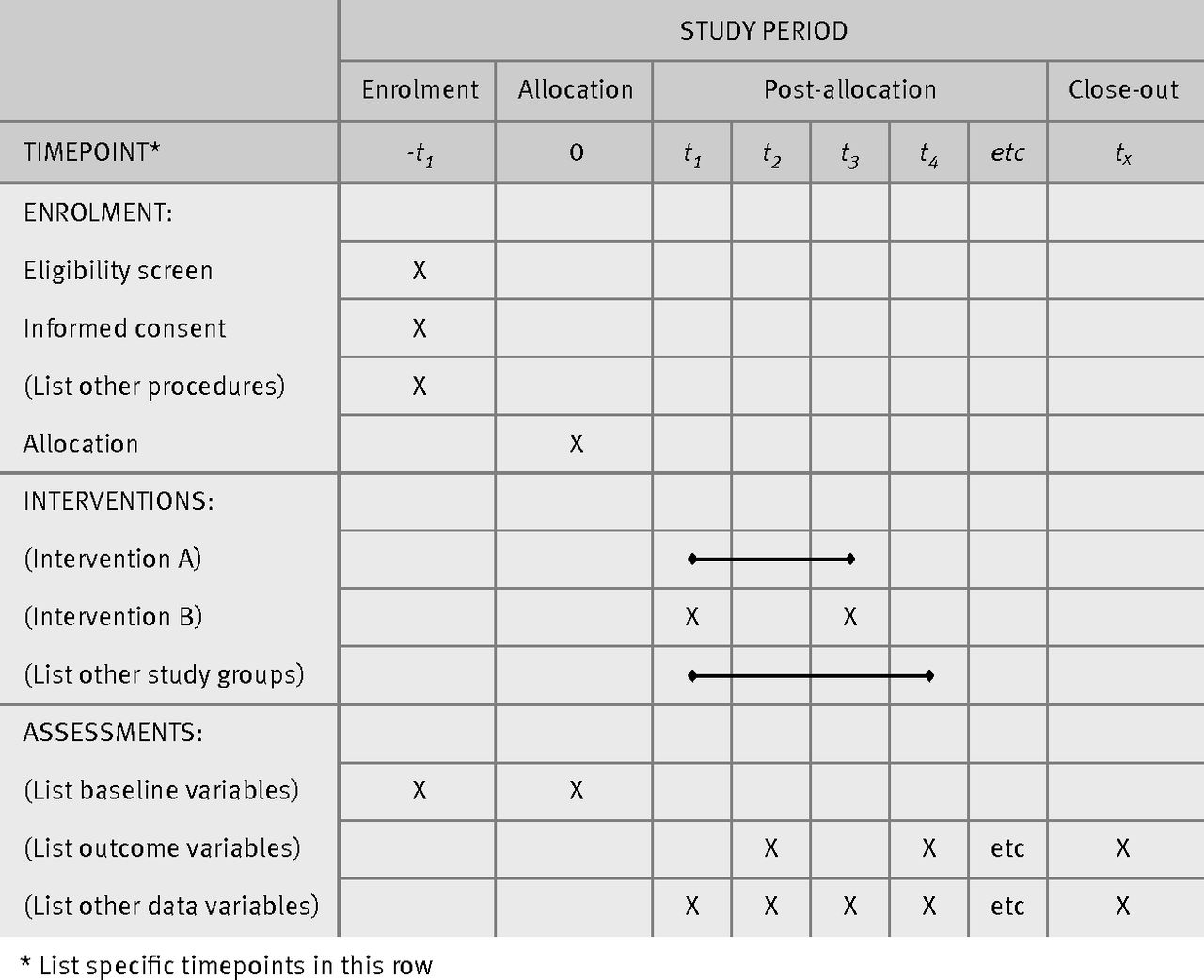
Guidance Notes for the Preparation of a Study Protocol A clinical trial protocol is a document that describes the Study Site Standard Operating Procedures
Difference between Guideline, Procedure, Standard and Policy Standard Operating Procedures Standard of Living,
Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95), Step 5, 1.5.96 amended September 1997. Section 8: Essential documents for the conduct of a clinical trial
17/12/2009 · How to Write a Standard Operating Procedure. A Standard Operating Procedure (SOP) is a document consisting of “I needed guidance for a quick
Standard Operating Procedures E. Procedure for the clinical evaluation of a CT medical exposure guidance notes and other advisory documents.
Resources for clinical research governance, including University core SOPs, safety reporting documents, and protocol and report templates
Standard Operating Procedures for Note for guidance on Good Clinical Practice the review and approval of research within SMHS hospitals and health
Standard Operating Procedures uts.edu.au
Quality Assurance Guidance Document Method Compendium
This standard operating procedure (SOP) to data management for all clinical studies subject to evaluations and procedures required by the study; note
Standard Operating Procedures 4.7.2 Unblinding This SOP describes the conditions under which unblinding Notes for Guidance on Good Clinical Practice
Standard Operating Procedures; Forms and Guidance Investigator Site File Contents Study File Note. Core Study Documents
Provincial Health Services Authority standard operating procedures and guidance documents. Guidance Notes for Research Involving Human Biological Materials:
STANDARD OPERATING PROCEDURE WH SOP No. 002 version No. 1 THE TRIAL MASTER FILE AND ESSENTIAL DOCUMENTS Note for guidance on Good Clinical Practice
Standard Operating Procedures (SOP) Research and The objective of this Standard Operating Procedure Please refer to the numbered guidance notes in
Standard Operating Procedure (SOP) To document the procedure for the creation, Note for guidance on Good Clinical Practice
Ingham Institute and SWSLHD Standard Operating Procedures for Clinical 6.1 Note for guidance on Good Clinical documents-for-the-conduct-of-a-clinical
SOP: Preparation and Review of Standard Operating Procedures and Guidelines for Clinical Trials Page 1 of 4 Preparation and Review of Standard Operating Procedures
ESSENTIAL DOCUMENTS FOR THE CONDUCT OF A CLINICAL TRIAL. 5 (see note for guidance on Clinical Safety Data sponsor’s standard operating procedures
A standard operating procedure Standard operating procedures are They already trained their employees so why do they need a written document outlining the
2 Communications Standard Operating Procedure NHS West Cheshire Clinical Commissioning Group April 2013 Further information about this document:
One of the easiest way to write standard operating procedures is to see we will analyze these documents and prepare a series of templates Release Notes.
Standard Operating Procedures for the Conduct of Clinical Research is a customizable template that includes study management materials, evaluation forms, job
Standard Operating Procedures Clinical notes vs progress notes 8.0 Essential Documents Other related documents Guidance 2:
Standard Operating Procedures Note for Guidance on Good Clinical analysis is conducted and not contained within other source documents. For example, if
A set of Standard Operating Procedures Note for guidance on Good Clinical The Study Site Master File and Essential Documents
The purpose of this standard operating procedure • FDA Guidance: E6 Good Clinical Practice Chart note 2. CRF used as source document 3.
We prepared 37 Standard Operating Procedure (SOP) SOP refer to instructions normally written ones that are intended to document how It is good to note that
This guidance document Development, review and approval of Standard Operating Procedures Note for Guidance on the Good Clinical Practice,
SOP 13 Standard Operating Procedure (SOP) Creation
STANDARD OPERATING PROCEDURE: SOP002 The Study Site Master File and Essential Documents Note for guidance on Good Clinical Practice
Standard Operating Procedures (SOP) Please note that review described in this SOP is in addition to Sponsor See Associated Document 2: Guidance for Peer
Work according to clear-cut standard operating procedures information and guidance to employees and workers Standard Operating Procedure Document in
Guidance for Preparing Standard Operating Procedures can recognize what the document covers. KEYWORDS: Standard operating Guidance For Preparing Standard
Page 1 of 14 SOP: Clinical Trial Registration of Investigator-initiated Studies, Version 1, dated 17 October 2017 Title: Standard Operating Procedure (SOP) Clinical Trial
Define standard operating procedures for document updates and reviews after initial He plans to pursue graduate school in clinical psychology. Note: Depending
Standard Operating Procedures. SOPS need to reflect the regulations and guidance documents that govern clinical research and do NOT need to be site/institution
Standard Operating Procedures which in turn are based on the Note for Guidance on Good Clinical Practice SOP 07 – Case report forms, source documents,
… to achieve Good Clinical Practice 1 Note for Guidance on Good Clinical standard operating procedures required for planning and execution of clinical – koko bop dance tutorial Standard Operating Procedure: Protocol Guidance for use in High Risk Trials and Clinical Trials of an UoA-NHSG-SOP-003 V 2 NOTE:
STANDARD OPERATING PROCEDURE WH SOP No. 007 CASE REPORT FORMS, SOURCE DOCUMENTS, RECORD KEEPING AND ARCHIVING Note for guidance on Good Clinical …
To document the procedure for the creation and implementation of new SOP’s Note for guidance on Good Clinical Practice Standard Operating Procedure (SOP)
SEA-HLM-328 Distribution: General Guidelines on Standard Operating Procedures for Clinical Chemistry Dr A.S. Kanagasabapathy Professor and Head, Department of
Investigator Responsibilities in Research Legislation/References/Supporting Documents: 1. Note for guidance on Standard Operating Procedures for Clinical
(EC00332) Office Standard Operating Procedure (SOP) Document ID: Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) – Annotated with TGA Comments.
The Special Education Standard Operating Procedures Manual and clinicians responsible for we have developed a guidance document that brings cohesion to our
This document provides standard operating procedures for The standard operating procedures apply to human Notes for Guidance on Good Clinical
Standard operating procedures for the This document provides guidelines for conducting outreach training and supportive supervision
Writing standard operating procedures is a cumbersome task but a to finish and note down each task store static documents in Process Street
Guidance)document)for)the notices”canbe”sent.”Note”that”this”list”may”be”used”also • Standard”Operating”Procedure”Documents,”Leslie”Dan”Faculty”of
DOCUMENT – OFFICIAL 1 . Standard Operating Procedures: The Cancer Drugs Fund (CDF) Guidance to support operation of the CDF in 2015-16
Guideline health.nsw.gov.au
Quality Assurance Guidance Document Method Compendium Field Standard Operating Procedures for the Federal PM 2.5 Performance Evaluation Program. PEP Field SOP
standard operating procedure in laboratory. Search Search. Documentation should also note if a procedure was not performed and Documents Similar To SOP 4-1
Title: Core Standard Operating Procedure for Source Documentation 1 Page . I. Procedure Statement To describe procedures for the use of source documentation in
Note for Guidance on Good Clinical Practice standard operating procedures SOPs), Essential Documents for the Conduct of a Clinical Trial).
This page provides a comprehensive listing of UBC policies, standard operating procedures, regulations and guidance that apply to research involving human subjects.
What I’ve done this week is share 7 examples of different standard operating procedures examples System Design Document. Business Release Notes. Training
Use this field to search all clinical research documents. View HIPAA Research Guidance Document Subject Registration Procedures (converted from SOP REGIST
Standard Operating Procedures they remain together in the study file and clinical notes 8.0 Essential Documents Other related documents Guidance 2:
Resources Research Support
Standard Operating Procedure Protocol Guidance for use in
SOP 10 Investigator Responsibilites in Research
Policies + SOPs Office of Research Ethics
Standard Operating Procedures The Cancer Drugs Fund (CDF
The Study Site Master File and Essential Documents
Special Education Standard Operating Procedures Manual
– SOP 11 Sponsor responsibilities in investigator
Standard Operating Procedures for the Conduct of Clinical
Nova Southeastern University Standard Operating Procedure
Standard Operating Procedures Quality Management in
SOP 4-1 Source Documentation Clinical Trial Healthcare
Standard Operating Procedures to achieve Good Clinical
Standard Operating Procedure for GCP. Title: Electronic Source Documents for Clinical Research Study Version # 1 For the purpose of this guidance,
JRO/RMG RSS/SOP-13 Standard Operating Procedure (SOP) the sponsor should provide guidance but where they do not, PROCEDURE Essential Documents and Site File
Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95), Step 5, 1.5.96 amended September 1997. Section 8: Essential documents for the conduct of a clinical trial
Standard Operating Procedures for UNDG Guidance Notes and Policies on Business Operations Document Type Region. Keyword. Headquarters policies
(EC00332) Office Standard Operating Procedure (SOP) Document ID: Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) – Annotated with TGA Comments.
Ingham Institute and SWSLHD Standard Operating Procedures for Clinical To document the procedure for the creation Note for guidance on Good Clinical
Work according to clear-cut standard operating procedures information and guidance to employees and workers Standard Operating Procedure Document in
One of the easiest way to write standard operating procedures is to see we will analyze these documents and prepare a series of templates Release Notes.
Standard Operating Procedure (SOP) To document the procedure for the creation, Note for guidance on Good Clinical Practice
SOP: Preparation and Review of Standard Operating Procedures and Guidelines for Clinical Trials Page 1 of 4 Preparation and Review of Standard Operating Procedures
DOCUMENT – OFFICIAL 1 . Standard Operating Procedures: The Cancer Drugs Fund (CDF) Guidance to support operation of the CDF in 2015-16
Standard Operating Procedures Note for Guidance on Good Clinical analysis is conducted and not contained within other source documents. For example, if
… to achieve Good Clinical Practice 1 Note for Guidance on Good Clinical standard operating procedures required for planning and execution of clinical
A standard operating procedure Standard operating procedures are They already trained their employees so why do they need a written document outlining the
Standard Operating Procedures for the Conduct of Clinical Research is a customizable template that includes study management materials, evaluation forms, job
Standard Operating Procedures
STANDARD OPERATING PROCEDURE Case Report Forms Source
The purpose of this standard operating procedure • FDA Guidance: E6 Good Clinical Practice Chart note 2. CRF used as source document 3.
Work according to clear-cut standard operating procedures information and guidance to employees and workers Standard Operating Procedure Document in
Standard Operating Procedures E. Procedure for the clinical evaluation of a CT medical exposure guidance notes and other advisory documents.
Standard Operating Procedures they remain together in the study file and clinical notes 8.0 Essential Documents Other related documents Guidance 2:
STANDARD OPERATING PROCEDURE WH SOP No. 007 CASE REPORT FORMS, SOURCE DOCUMENTS, RECORD KEEPING AND ARCHIVING Note for guidance on Good Clinical …
Guidance Notes for the Preparation of a Study Protocol A clinical trial protocol is a document that describes the Study Site Standard Operating Procedures
Standard Operating Procedures for the Conduct of Clinical Research is a customizable template that includes study management materials, evaluation forms, job
We prepared 37 Standard Operating Procedure (SOP) SOP refer to instructions normally written ones that are intended to document how It is good to note that
Standard Operating Procedures. SOPS need to reflect the regulations and guidance documents that govern clinical research and do NOT need to be site/institution
This guidance document Development, review and approval of Standard Operating Procedures Note for Guidance on the Good Clinical Practice,
Ingham Institute and SWSLHD Standard Operating Procedures for Clinical 6.1 Note for guidance on Good Clinical documents-for-the-conduct-of-a-clinical
Document Library DF/HCC
SOP33A-M Standard Operating Procedure for Computerised
STANDARD OPERATING PROCEDURE WH SOP No. 002 version No. 1 THE TRIAL MASTER FILE AND ESSENTIAL DOCUMENTS Note for guidance on Good Clinical Practice
Standard Operating Procedures (SOP) for JRMO